Required for multiple aspects of GABA neuron identity 7,8. These studies also revealed the first cation-selective GABA receptor 9. This review will discuss first the strategy used to identify proteins required for GABA neurotransmission in C. Elegans and then the impact that their discovery has had on our understanding of GABA function. Corresponding sets of motor neurons analogous to those in C. Elegans are found in the larger nematode Ascaris lumbricoides (Stretton et al., 1978; Johnson and Stretton, 1980). Eph receptor protein-tyrosine kinases are among the oldest known animal receptors and have greatly expanded in number during vertebrate evolution. Their complex transduction mechanisms are capable of bidirectional and bimodal (multi-response) signaling. Eph receptors are expressed in almost every cell type in the human body, yet their roles in development, physiology, and disease are.
C Elegans Neuron Map
For the present study, Rockefeller’s Xin Jin and colleagues described a form of aversive imprinting in their C. elegans: newly hatched nematodes exposed to Pseudomonas aeruginosa PA14 or toxin-emitting Escherichia coli BL21 established a long-term olfactory aversion to it. Animals that experienced the pathogen immediately after hatching were able to synthesize and maintain the aversive memory for the whole of their four-day lifespans, while animals trained in adulthood only retained the aversive memory for up to 24 hours.
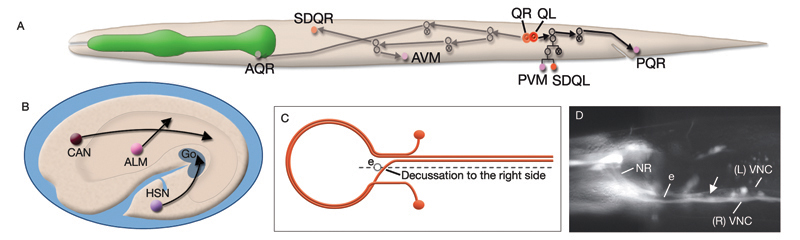
C. elegans has an exceedingly compact nervous system with only 302 neurons (compared with 250,000 in the fruit fly Drosophila and 86 billion in humans). To understand which specific neurons were involved in the different stages of aversive imprinting, the researchers selectively and reversibly deactivated individual brain cells. Silencing the neurons AIB or RIM during the memory-formation phase prevented learning in the newly hatched nematodes, but silencing these same cells during memory retrieval showed no effect. The opposite was true of the neurons AIY and RIA: these cells could be silenced during memory formation with no effect, but were indispensable during memory retrieval.
The principle of separate neural circuits for memory formation and retrieval is far from unique to C. elegans. It was shown in humans through cases such as the famous patient “H.M.” who, following surgery that removed his medial temporal lobe, was able to retrieve old declarative memories but unable to form new ones.
“[The] idea that the transient learning signal would later be dispensable at the time of memory goes back as far as Pavlov. We’re just developing the idea at a different level of resolution to map it onto a physical site and not just a conceptual site,” said study coauthor Cori Bargmann. “It’s a surprise all over again that you can actually implement this in such a compact, little brain.”
Of course, memory formation and retrieval circuits must communicate with each other for learning to occur. The researchers found one molecular bridge between the circuits in the neurotransmitter tyramine, a homologue of adrenaline in mammals. Tyramine was released by the memory-formation neuron RIM and detected by the memory-retrieval neuron AIY; the neurotransmitter alone could replace the requirement for RIM activity in the C. elegans learning process.
“We often describe phenomenology and then speculate about the underlying machinery, but research in C. elegans—and especially this particular group—have really taken it to a different level, describing behaviors in great mechanistic detail,” said Harvard neurobiologist Bence Ölveczky, who was not involved in the research.
The present work is “really just the tip of the iceberg,” Jin told The Scientist. “Thanks to rapidly evolving modern genetics, neuromanipulation and imaging tools, we’re able to study causal effects of animal behaviors at a new level of precision. It’s an exciting time.”
X. Jin et al., “Distinct circuits for the formation and retrieval of an imprinted olfactory memory,” Cell, doi:10.1016/j.cell.2016.01.007, 2016.
ABOVE: © ISTOCK.COM
HEITIPAVES
C.elegans Movement
Biologists have mapped out the neural connections between each neuron and with organs in the worm Caenorhabditis elegans, in a study published in Natureon July 3. The connectomes trace 385 neurons in the male worm and 302 neurons in the hermaphrodite worm.
C Elegans Model
“It’s a major step toward understanding how neurons interact with each other to give rise to different behaviors,” says coauthor Scott Emmons, a developmental biologist at the Albert Einstein College of Medicine in New York, to The New York Times.
Previous research by Sydney Brenner’s group at the Salk Institute in the 1970s mapped the connectome of the hermaphrodite sex of C. elegans, but it was incomplete. “We just had fragments of the worm’s wiring,” says Emmons. This study adds the male connectome and provides more complete connections for both sexes. “The new connectomes provide much more comprehensive information than the old data sets did,” Aakanksha Singhvi of the Fred Hutchinson Cancer Research Center who was not involved in the study tells the Times.
In future research, “the ability to link specific neurons with specific behaviors will. . . allow us to really establish the extent to which wired and wireless circuits contribute to specific behaviors,” Piali Sengupta of Brandeis University who was no involved with the study tells The Washington Post. For example, schizophrenia and bipolar disorder “may be connectopathies. That is, they’re due to abnormal or faulty or incorrect wiring somewhere in the nervous system,” says Emmons to the Post.
Experts say that they need more connectomes of C. elegans and other species. “We need to have many more examples of individual C. elegans,” says Douglas Portman of Rochester University who was not involved with the work to the Post.
C Elegans Development
Chia-Yi Hou is an intern at The Scientist. Email her at chou@the-scientist.com.